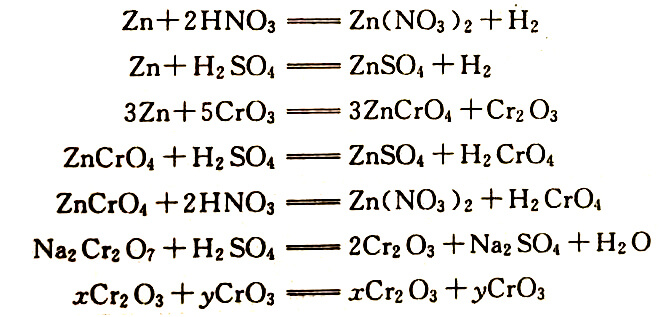What is Chromated Treatment?
Chromated treatment involves immersing a metal object in a solution primarily composed of chromic acid or dichromate, resulting in the formation of a protective film on the metal surface. This post-treatment process is commonly used for zinc and cadmium coatings. The following sections will break down the basics, the chemical process, and the applications of chromate treatment.
The Basics of Chromate Treatment
What is Chromated Treatment?
Chromate treatment is a chemical process where a metal surface is treated with a chromate solution. This treatment forms a thin, protective film that prevents rust and corrosion, making it an essential step in metal finishing for enhancing the durability and lifespan of the coated metals.
How Does Chromated Treatment Work?
The treated metal is immersed in a chromate or dichromate solution. The chromate reacts with the metal surface, forming a protective, passive layer composed of trivalent and hexavalent chromium compounds and water. This layer prevents the formation of white corrosion products that can damage the metal coating.
Chemical Composition and Process
Chromated Compounds
Chromate treatment primarily uses hexavalent chromium compounds, including:
- Chromic Acid (H2CrO4)
- Potassium Chromate (K2CrO4)
- Sodium Chromate (Na2CrO4)
- Potassium Dichromate (K2Cr2O7)
- Sodium Dichromate (Na2Cr2O7)
These compounds are responsible for the formation of the protective chromate film.
The Chemical Reaction
During the chromate treatment, the metal surface reacts with the chromate solution, leading to the formation of a stable chromate film. This film is typically very thin but provides significant protection against corrosion.
Applications of Chromate Treatment
Chromate treatment is used across various industries for several purposes:
- Surface Protection: It is widely used for protecting aluminum, magnesium alloys, and zinc-coated metals from corrosion.
- Enhancing Aesthetics: The treatment improves the appearance of metal surfaces, providing a uniform and appealing finish.
- Improving Adhesion: Chromate coatings enhance the adhesion of subsequent layers of paint or other coatings.
- Surface Hardening: In some cases, the treatment modifies the surface structure, increasing hardness and wear resistance.
Advantages of Chromated Treatment
- Corrosion Resistance: Provides excellent protection against rust and corrosion.
- Enhanced Adhesion: Improves the adhesion of paints and other coatings.
- Aesthetic Improvement: Offers a uniform, attractive finish.
- Increased Surface Hardness: In certain applications, it enhances the surface hardness and wear resistance.
Disadvantages and Environmental Concerns
- Toxicity: Hexavalent chromium compounds are toxic and pose significant health risks.
- Environmental Impact: Strict regulations govern the use of chromate due to its environmental impact, leading to increased costs and alternative treatment developments.
Common Chromate Compounds
Some commonly used chromate compounds in chromate treatment include:
- Sodium Chromate (Na2CrO4)
- Potassium Chromate (K2CrO4)
- Lead Chromate (PbCrO4)
- Ammonium Chromate ((NH4)2CrO4)
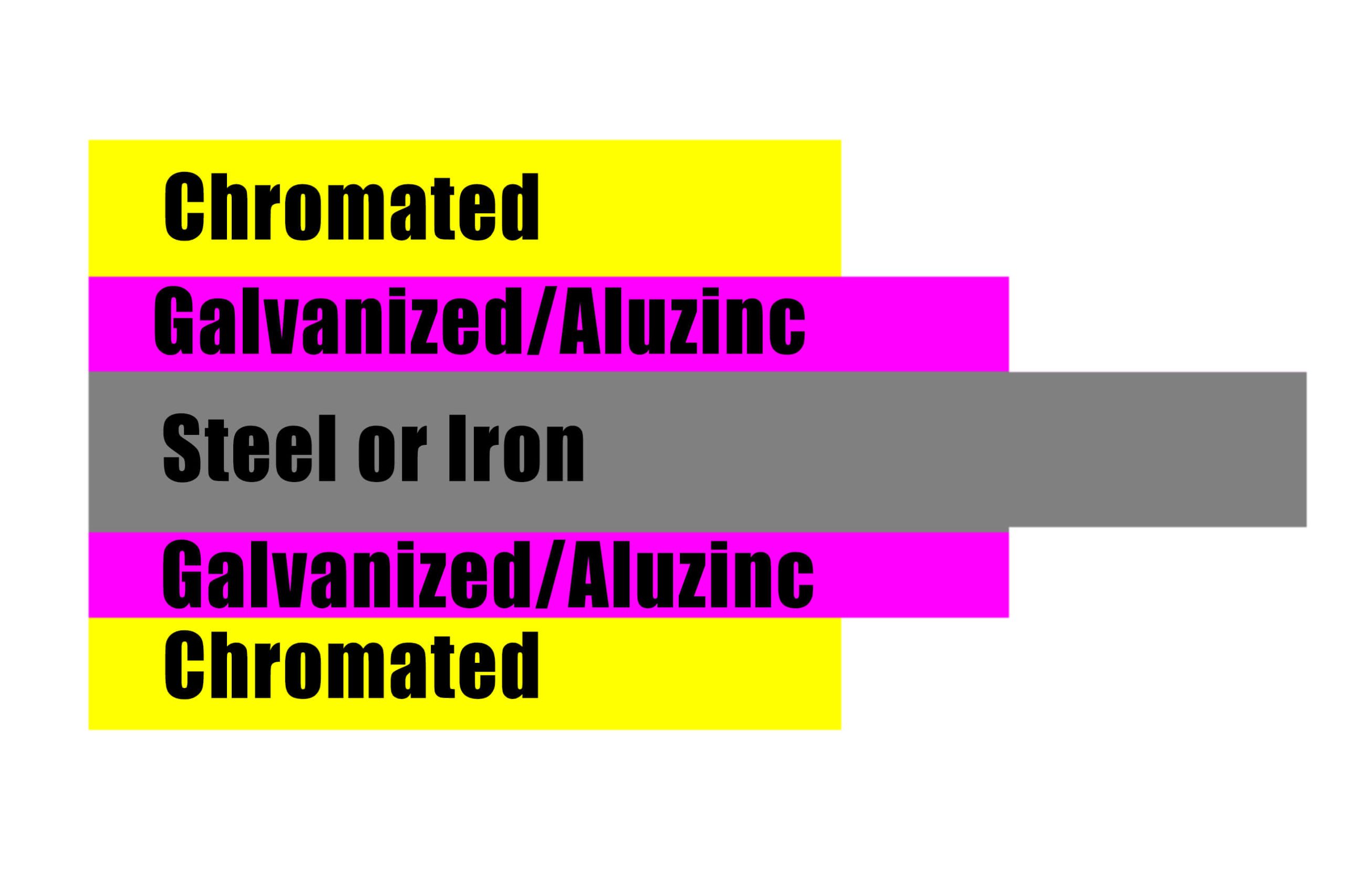
The chemical reactions that occur during chromate treatment are as follows (in the case of a galvanized layer):
Application of chromate
The main purpose of chromate treatment is to form a rust-proof protective film, improve the appearance and gloss, and improve its decorative performance, but it does not have such an effect on any coating. Generally speaking, zinc plating and cadmium plating can form a good rust-proof covering film, while copper plating, silver plating, etc. can only prevent discoloration. Even galvanizing has a good effect on zinc cyanide electroplating, but the effect on zinc sulfate electroplating or molten zinc electroplating composed of old liquid is not ideal. Cadmium plating is also suitable for cadmium cyanide electroplating, but is less effective for cadmium boron fluoride electroplating. The uses of chromate treatment are shown in Table 2.
| Application | Remark |
| Improve corrosion resistance | The most widely used method is to treat galvanized or cadmium-plated items with chromate to prevent the coating from being exposed to the outside world and producing white corrosion products.a |
| Improve gloss | Use the chemical grinding effect of concentrated chromate solution to improve the gloss of galvanized or cadmium plated layers |
| Prevents discoloration | Silver or copper plating is prone to discoloration, and it is not easy to change color after chromate treatment, and anti-discoloration agents using chromate have been developed |
| Metal coloring | Chromate coating (colorless) is easy to adsorb dyes, so chromate treatment can be used for metal coloring |
| Pre-coating treatment | The coating is easy to attach to the chromate coating. In order to improve the adhesion and rust resistance of the coating, chromate treatment can be used. |
Chromated Treatment Process Flow and Solution Formula
A. Process flow of chromated treatment
There are three main methods listed in Table 3 for chromate treatment, and the dilute nitric acid in Table 3 is immersed in about 0.5% nitric acid solution to neutralize the remaining plating solution (alkaline) on the surface of the coating that cannot be washed off with water, and dissolve the surface layer of the coating to expose its luster. Placed in air is to be kept in the air for a few seconds when the chromate tank is moved to the water bath. When the coating is immersed in concentrated chromic acid, it is chemically ground in the liquid to improve the gloss, but does not generate a chromate coating, and when it is removed from the treatment solution and placed in the air, a film is formed, and the chromate solution attached to the surface of the coating reacts with the coating to form a coating. If the time is long, a thick film will be formed, and if the time is short, a thin film will be formed.
To obtain a glossy and colorless chromate film, it needs to be immersed in an alkali solution (Na2CO3 60g/L, 30~60℃). When immersed in the alkali solution, hexavalent chromium dissolves into the alkali solution and decolorizes into a shiny and colorless chromate film. After the hexavalent chromium is dissolved, the corrosion resistance of the film decreases, but it has a metallic luster, which increases its appearance. The main components of the membrane are trivalent chromium and water.
To obtain a film that is both glossy and colorful, the two-liquid method can be used. First use a concentrated chromate solution to chemically grind, and then immerse in a low-concentration chromate solution that has no chemical grinding effect. A chromate coating is formed in a low-concentration chromate solution without chemical polishing and without improving the gloss.
B. Chromated treatment composition
The basic formula of the treatment solution for passivation treatment of zinc and cadmium coatings. Among them, formula 1 is the traditional Cronak di-chromate type. The soaking time of this solution is short, only about 10s, and it is very sensitive to time.
The film formed is very thin and has an interference-type rainbow. Over time, the color of the film changes from light to dark. It turns green at 5s and brown at 15s. Long-term treatment will powder the film and it is easy to fall off. When the film is not yellow, it can be bleached with 60g/L sodium phosphate solution, which is beautiful blue-white or white after bleaching.Close to transparency. Some customers like to use black.
Chromate treatment solutions are divided into two types: high concentration and low concentration. Those with chromic acid concentration above 80g/L are called high concentration, and those below 80g/L are low concentration. High-concentration chromic acid solutions have chemical grinding effects, but low-concentration ones do not.
To obtain a shiny passivation film, useFor high-concentration liquid treatment, the cost of using high-concentration liquid is high, and sewage treatment is more difficult. The cost of low-concentration liquid is lower and the degree of harm is smaller. Heavy chromate solution is also a high-concentration, low-concentration chromate solution.
Factors affecting the quality of chromated passivation film
A. Composition of chromated coating
Chromate film on zinc plating surface was analyzed. Chromium is the most, followed by water, followed by hexavalent chromium, in addition to sulfate, zinc, sodium and other components, it can be seen that the chromate film is mainly composed of chromium, hexavalent chromium and water, the chemical formula is Cr₂O₃·CrO₃·xH₂O, water is crystalline water form. Cr6 + and Cr3 + are the main factors affecting the corrosion resistance of the coatings, and Cr6 + plays a major role in the corrosion resistance. Therefore, in order to obtain a passivation film with good corrosion resistance, it is necessary to generate a chromate film with high hexavalent chromium content.
B. Effect of Time in Air on Corrosion Resistance
If placed in the air for a long time, the amount of hexavalent chromium increases, the coating thickness increases, and the corrosion resistance also increases. However, sometimes the thickening of the film will reduce the adhesion and the film will fall off, so attention must be paid. In general, when immersed in a low concentration of chromate solution for a long time, the adhesion is weakened. When 30mL/L glacial acetic acid is added to the chromate solution, the adhesion of the film is enhanced. When potassium permanganate is added to the chromate solution, the adhesion can also be improved. When the metal concentration (zinc, cadmium, etc.) in the chromate solution increases, the adhesion of the film decreases.
C. Effect of Aging on Corrosion Resistance
The corrosion resistance of chromate coating is weak at the beginning of production, and the corrosion resistance increases with the increase of storage time, and the hardness and adhesion of the film also tend to have this effect. Therefore, corrosion resistance test should be carried out at least 24 hours after the film formation. However, when heated and dried at a temperature of 60 ° C or less, the corrosion resistance can be increased in a short time.
D. Effect of Drying Temperature on Corrosion Resistance
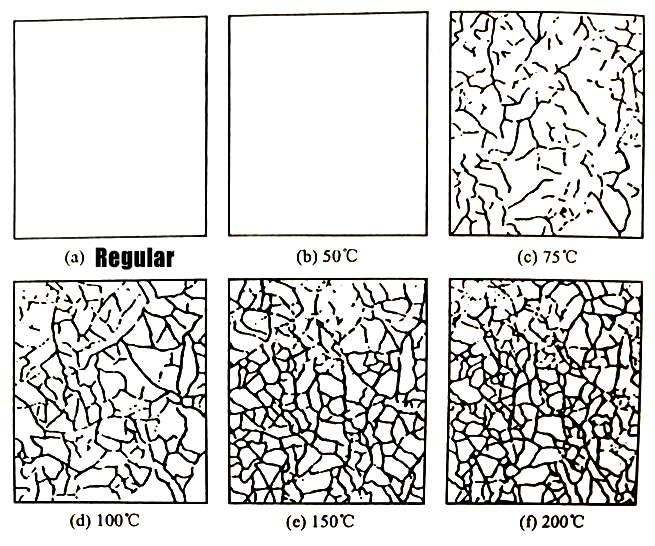
The drying temperature after the chromate film is formed has a great influence on the corrosion resistance of the film. It should be dried below 60°C in the afternoon. The corrosion resistance decreases above 70°C, and the corrosion resistance drops sharply at 80°C. Because when drying above 70°C, cracks appear in the chromate film and the corrosion resistance decreases.
The reason for the cracks is that the chromate coating contains a certain amount of moisture. When the temperature reaches above 70°C, the water begins to break away from the film layer, so Dry and cracked. the display of the zinc coating after chromate treatment, heating and drying at a temperature from 50 to 200°C, and then observing the surface of the film with a microscope. it can be seen that the temperature does not change at 50°C. In rupture, there are cracks at 75℃, the higher the temperature, the more cracks.
How to Find Galvanized Steel Suppliers?
If you want to find a reliable steel maufacturer in China, we will be happy to help you with a free consultation. I wrote an article about 5 Tips to Help You Select Galvanized Steel Coil Manufacturers in China. You can refer to this method to find good suppliers.